TSO is a new drug, taken by mouth, that contains microscopic eggs of the pig whipworm parasite. It acts as a natural regulator of the immune system, by controlling T-cells, a group of white blood cells, and cytokines, signalling proteins that deal with inflammation, among other things.Seems interesting. Reminds me of helminthic therapy -- although doesn't sound like the worms stick around past two weeks. Hints to a lack of diversity of bacteria and other organisms in our gut.
In a pig, the eggs grow into whipworms and reproduce, without causing harm to the animal. In a human, the same eggs live no more than two weeks. But in that time, they appear able to influence the host immune system and stop it attacking tissue and organs.
Tuesday, August 28, 2012
Pig Parasite To Be Trialled As Treatment For Crohn's Disease
Biologics for Inflammatory Bowel Disease Increase Risk for Melanoma
Patients with IBD had a higher incidence of melanoma than controls; this association was significant in patients with Crohn disease (incidence rate ratio, 1.45) but not in patients with UC (IRR, 1.13). Researchers observed a trend of increasing risk for melanoma in patients with IBD over time. In a nested case-control analysis, any use of a biologic anti–tumor necrosis factor (anti-TNF) agent was associated with an almost twofold increased risk for melanoma, unlike thiopurines or 5-aminosalicylic acid (5-ASA) medications, for which no associations were observed. The risk for NMSC was increased by almost twofold in patients who used any thiopurine, but was not significantly increased in patients who used anti-TNF agents.Makes sense that there are side-effects of using biologics.
Tuesday, July 13, 2010
Why some people with IBD may not respond to corticosteroids
Currently, those with lupus and other autoimmune diseases, commonly treat the condition with corticosteroids to suppress their overactive immune system and prevent it from attacking healthy tissues which can result in symptoms such as inflammation, pain and organ damage.
These steroid treatments work by killing certain immune system cells, including plasmacytoid dendritic cells (PDCs) that overproduce type 1 interferons, an immune system substance that contributes to lupus and other autoimmune diseases. However, unlike other conditions, steroid treatments are not as effective against these cells in those with lupus.
By largely studying children with systemic lupus erythematosus (SLE), BRI scientists in collaboration with scientists at Dynavax in Berkeley, CA, were able to solve the mystery behind the resistance. They determined that two immune system proteins known as toll-receptor 7 (TLR7) and toll-receptor 9 (TLR9), cause an activation of PDCs—the very cells steroids target—negating the effects of treatment. BRI scientists reported their findings in the June issue of the journal Nature.
A similar resistance mechanism might be at play with people with IBD (and other autoimmune diseases). It hasn't been proven yet obviously, but it's certainly something to consider when you're working with your doctor to deal with a serious flare-up. The traditional prednisone or budesonide (Entocort) may not work for you simply because your body works against the mechanism of the drugs.
Thursday, July 8, 2010
Two new drugs that could be used for autoimmune and inflammatory diseases
Monday, June 21, 2010
Prostaglandin D2 a potential treatment for Ulcerative Colitis
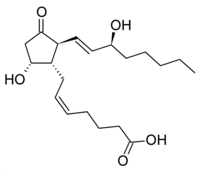 A recent study may suggest a new treatment for ulcerative colitis. The study, to be published in the journal Proceedings of the National Academy of Sciences, found that people with ulcerative colitis that have been in remission for a long time-period have higher levels of Prostaglandin D2 than those that don't. It's unclear whether the higher levels of the chemical are a result of being in remission or the cause of the remission, but the study is interesting nonetheless as it suggests follow-on research.
A recent study may suggest a new treatment for ulcerative colitis. The study, to be published in the journal Proceedings of the National Academy of Sciences, found that people with ulcerative colitis that have been in remission for a long time-period have higher levels of Prostaglandin D2 than those that don't. It's unclear whether the higher levels of the chemical are a result of being in remission or the cause of the remission, but the study is interesting nonetheless as it suggests follow-on research.
FDA requires cancer warning on TNF blockers (old news)
 I've thought for a while that TNF blockers and other immunosuppressive drugs may actually be counterproductive in the long-run in treating Crohn's Disease and IBD. The point of TNF-alpha in the immune system is to promote the fight against tumors (TNF = tumor necrosis factor). What happens if you suppress that immune response? Your body may be missing tumors that it should be fighting. A recent article prompted me to take a look at the FDA warnings for TNF-alpha blockers. Apparently, the FDA started requiring cancer warnings on TNF blockers back in August of 2009 -- here's the FDA press release. I think dozens of cancer cases in children taking TNF-blockers prompted the FDA to add the box warning.
I've thought for a while that TNF blockers and other immunosuppressive drugs may actually be counterproductive in the long-run in treating Crohn's Disease and IBD. The point of TNF-alpha in the immune system is to promote the fight against tumors (TNF = tumor necrosis factor). What happens if you suppress that immune response? Your body may be missing tumors that it should be fighting. A recent article prompted me to take a look at the FDA warnings for TNF-alpha blockers. Apparently, the FDA started requiring cancer warnings on TNF blockers back in August of 2009 -- here's the FDA press release. I think dozens of cancer cases in children taking TNF-blockers prompted the FDA to add the box warning.Thursday, June 17, 2010
Why Pregnancy Pushes Autoimmune Diseases into Remission
 A friend of a friend of mine has had Crohn's for many years. But when talking to her, she mentioned that during her pregnancy all her Crohn's symptoms completely subsided and she was in "remission". Why? This apparently is a common phenomenon for pregnant women with autoimmune diseases -- during pregnancy their diseases go into remission.
A friend of a friend of mine has had Crohn's for many years. But when talking to her, she mentioned that during her pregnancy all her Crohn's symptoms completely subsided and she was in "remission". Why? This apparently is a common phenomenon for pregnant women with autoimmune diseases -- during pregnancy their diseases go into remission.In his search to explain the phenomenon, Dr. Petty knew to look for a metabolic pathway ormechanism with two characteristics. It had to "dial down" the intensity of the normal immune response, an action needed so that a pregnant woman does not reject the fetus, which has proteins from the father that are "foreign" to the mother. At the same time, such a mechanism must support cell growth needed by the developing fetus.
The activity of the enzyme pyruvate kinase–and its product, pyruvate–fills both roles: promoting cell growth while modifying the immune response. Because pyruvate kinase activity is depressed duringpregnancy, cell metabolism supports an increased production of lipids, carbohydrates, amino acids, and other substances that support cell growth.
Thursday, May 6, 2010
Anti-TNF Decreases Presence of MAP
 I only saw the abstract of this one. There was a study that found that taking anti-TNF drugs, in this case infliximab (i.e. Remicade), reduced the occurrence of mycobacterium avium paratuberculosis (MAP) bacteria in people suffering from Crohn's. Specifically, they just measured the change in MAP antibodies in the blood, but it would suggest that Remicade supported the body's fight against any MAP infection. I've had a couple posts on MAP, so I like to monitor research in this area. Interesting finding that supports the efficacy of these drugs (although admittedly I don't take them).
I only saw the abstract of this one. There was a study that found that taking anti-TNF drugs, in this case infliximab (i.e. Remicade), reduced the occurrence of mycobacterium avium paratuberculosis (MAP) bacteria in people suffering from Crohn's. Specifically, they just measured the change in MAP antibodies in the blood, but it would suggest that Remicade supported the body's fight against any MAP infection. I've had a couple posts on MAP, so I like to monitor research in this area. Interesting finding that supports the efficacy of these drugs (although admittedly I don't take them).
Thursday, December 24, 2009
A Potential Stem Cell Treatment for IBD from Pfizer


Pfizer announced some news this week that could result in a stem cell treatment for inflammatory bowel disease (IBD), including Crohn's. On Monday, Pfizer struck a development and commercialization agreement with Athersys, Inc., a Cleveland biotech company. Pfizer plans to develop a therapy for IBD based on MultiStem, the Athersys adult stem cell product line.
MultiStem consists of a special class of human stem cells that have the ability to express a range of therapeutically relevant proteins and other factors, as well as form multiple cell types. Factors expressed by MultiStem have the potential to deliver a therapeutic benefit in several ways, such as the reduction of inflammation, protection of damaged or injured tissue, and the formation of new blood vessels in regions of ischemic injury. These cells exhibit a drug-like profile in that they act primarily through the production of factors that regulate the immune system, protect damaged or injured cells, promote tissue repair and healing and most or all of the cells are cleared from the body over time.
Though the cells have the potential to differentiate into a variety of cell types, in certain indications the primary mechanism of MultiStem appears to be the production of a physiologically relevant and complex set of therapeutic molecules in response to the local environment. In the initial indications Athersys is pursuing, the cells appear to minimize the inflammatory reaction that occurs in response to ischemic events (such as myocardial infarction or stroke) or the anti-host immune reaction seen in graft vs. host disease (GvHD), and promote healing and recovery. Unlike traditional pharmaceuticals, MultiStem cells are dynamically regulated, and have the potential to respond to signals of inflammation or tissue damage in multiple ways. Potential mechanisms of benefit include protection of damaged or injured cells, reduction of inflammation, stimulation of new blood vessels, and the recruitment of other cell types to promote tissue repair and healing.
MultiStem is being tested in several conditions, but Pfizer's license is specifically for the treatment of inflammatory bowel disease (IBD), a group of conditions that includes ulcerative colitis and Crohn's disease. The license is only costing Pfizer $6 million up front because the technology is still relatively unproven, having not entered clinical trials for IBD yet. Athersys can get milestones of up to $105 million and royalties as the drug passes through clinical trials and is commercialized.
Pfizer will pay for the phase 1 and 2 trials. Then, if it gets that far, Athersys will have the option of co-developing the drug -- sharing profits and losses -- or letting Pfizer proceed on its own and take the milestones and royalty payments.
Unlike traditional stem cell companies like Geron (Nasdaq: GERN) that are developing stem cells to regenerate tissue, MultiStem uses donated bone marrow cells to produce a product that promotes healing of the tissue through cell signaling. Essentially it has a more drug-like profile as the stem cells are cleared from the body.